Abstract
Background:
Venetoclax, a small-molecule inhibitor of B cell leukemia/lymphoma-2, in combination with hypomethylating agents (HMA) has shown improved efficacy and survival benefit compared to HMA alone (DiNardo et al, 2020) in elderly/unfit patients with acute myeloid leukemia (AML). Since FDA-approval of this regimen for elderly/unfit AML patients, it is frequently utilized both in the upfront and relapsed/refractory setting. Cardiac events with venetoclax are not well described. In the VIALE-E trial, which included patients ineligible for standard induction chemotherapy due to congestive heart failure or stable angina, 15% of patients receiving azacitidine plus venetoclax experienced atrial fibrillation as a serious adverse event, vs. 1% in the azacitidine plus placebo group (DiNardo et al, 2020). Our objective was to provide an estimate of the prevalence of and a description of all cardiac events that occurred in AML patients undergoing treatment with venetoclax + HMA.
Methods:
170 consecutive patients with AML who received venetoclax +HMA (azacitidine or decitabine) outside the context of a clinical trial between 1/2017-11/2020 at the Mayo Clinic were included. Patients received venetoclax + HMA either as upfront treatment or for relapsed/refractory disease. Patients with relapse following allogeneic stem cell transplant were excluded. We evaluated all cardiac events that occurred while treatment with venetoclax + HMA was ongoing. Baseline patient and treatment characteristics were compared using the Mann-Whitney U-test and the Fisher's exact test. All statistics were computed using EZR (Version 1.53).
Results:
1. Patient characteristics
A total of 170 patients who received venetoclax + HMA (median age 69 years [range 17-91], 63% males) were included. ELN risk category was either adverse risk (48%, 82/170) or intermediate risk (48%, 82/170) in the majority of patients. 64% (109/170) of patients received venetoclax + HMA as upfront treatment. Characteristics including age, ELN cytogenetic risk, cardiovascular risk factors, and upfront vs relapsed therapy were similar among patients with or without cardiac events. The only exception was a higher incidence of CEBPA mutation amongst those with cardiac events (12% vs 2%, p=0.03). The majority (83%, 141/170) of patients underwent an echocardiogram prior to initiation of therapy.
2. Cardiac events
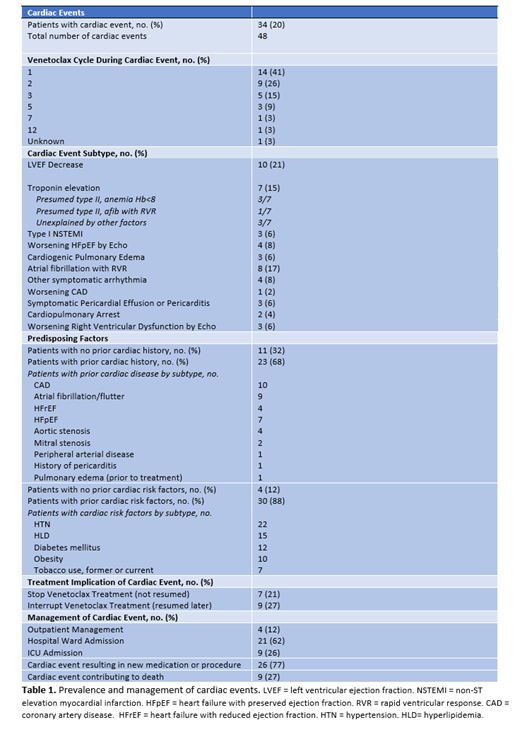
Of 170 patients treated with venetoclax + HMA during the study period, 34 (20%) patients experienced a total of 48 cardiac events. Of patients experiencing cardiac events, 32% (11/34) had no pre-existing cardiac disease and 12% (4/34) had no cardiovascular risk factors (Table 1). The majority of events occurred early in treatment course: 41% during cycle 1, 26% during cycle 2 and 15% during cycle 3 (Table 1).
The most frequently occurring cardiac event (21%, 10/48 events) was a decrease in left ventricular ejection fraction on echocardiography, which was associated with symptoms in all ten patients. Second most frequent was atrial fibrillation with rapid ventricular response at 17% (8/48 events), followed by troponin elevation without electrocardiogram changes at 15% (7/46 events). Of patients with troponin elevation, 57% (4/7 events) occurred in the setting of another inciting factor such as severe anemia, while 43% represented a troponin elevation without explanation (Table 1). Other cardiac events included heart failure with preserved ejection fraction (n=4), other symptomatic arrhythmia (n=4), and symptomatic pericardial effusion or pericarditis (n=3). In addition, 2 of 34 (6%) patients experienced fatal cardiopulmonary arrest.
The majority (88%) of cardiac events required either inpatient admission (62%, 21/34 patients) or intensive care unit (ICU) care (26%, 9/34 patients). 77% of patients required new cardiac medications or procedural intervention (n=4). In 27% of cases (9/34 patients), the cardiac event directly contributed to death (Table 1).
Conclusions:
Cardiac complications were observed in one-fifth (20%) of AML patients treated with venetoclax + HMA, despite the absence of preexisting cardiac disease in a third of cases; moreover 27% of events were fatal. Further comparative studies are required to identify salient clinical features predictive of cardiac complications in these patients.
Al-Kali: Novartis: Research Funding; Astex: Other: Research support to institution. Litzow: Astellas: Research Funding; Amgen: Research Funding; Omeros: Other: Advisory Board; AbbVie: Research Funding; Actinium: Research Funding; Pluristem: Research Funding; Jazz: Other: Advisory Board; Biosight: Other: Data monitoring committee. Patnaik: Kura Oncology: Research Funding; StemLine: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract